臨床デザイン・臨床統計ソフト nQuery
製品概要 | ユーザー紹介 | システム条件 | 製品カタログ
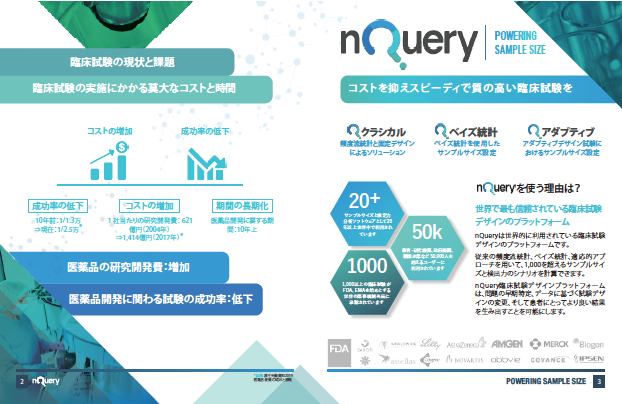
臨床試験の成功確率向上のカギとなるのは試験デザインです
nQueryは臨床試験における第I相〜第IV相すべてのフェーズと試験デザイン(固定デザインと適応的デザイン)をカバーする臨床統計ソフトウェアです。
仮説検定や信頼区間など頻度主義に基づく従来のデザインと、ベイズ統計を用いたアダプティブ・デザインに対応した統計解析機能を備えており、臨床試験のパフォーマンスを高めるプラットフォームとして多くの企業・団体・機関に導入されています。
固定デザインからアダプティブデザインへ
コストを抑えスピーディで成功率の高い臨床試験を実現する革新的なアダプティブデザイン(adaptive design)の利用が世界的に広がってきています。nQueryは臨床試験における第I相から第IV相のすべてのフェーズをカバーしており、臨床試験のパフォーマンスを高めるプラットフォームとして多くの企業・団体・機関に導入されています。
アダプティブデザインを用いた臨床試験
アダプティブデザインは特にオンコロジー分野で採用されていましたが、2019年11月にアダプティブデザインに関するFDA のガイダンスAdaptive Designs for Clinical Trials of Drugs and Biologicsが発出されたこともあり急速に発展しました。 COVID-19パンデミックにおけるグローバルな共同治験でも多く用いられるなど、アダプティブデザインは国際的に更なる広がりを見せています。
世界中で利用されている臨床試験デザインソフト
nQueryは臨床試験のパフォーマンスを高めるプラットフォームとして、1995年の販売開始以来、多くの企業・団体・機関に導入されています。
20+ Years
サンプルサイズと検定力分析ソフトウェアとして20年以上、世界中で利用されています。
50k Users
教育機関、研究機関、政府機関、製薬企業など、50,000人を超えるユーザーに利用されています。
Successful Trials
食品医薬品局(FDA)、欧州医薬品庁(EMA)を始めとする世界の薬事規制当局に認められています。
nQueryが利用された論文・ジャーナル
臨床試験だけではなく、多くの非臨床薬理試験(in vivo, in vitro)でもサンプルサイズ設計や検出力計算に利用され、nQuery で算出された結果は多くの論文で引用されています。
- TRPA1 as Target in Myocardial Infarction
-
"Assuming the previously observed standard deviation, 17 animals/group were necessary to detect the relevant mean difference of 15% with a power of 80%, accepting the probability of a type I error of 5% after correction for multiplicity. Estimation of necessary sample size was performed with nQuery (Statstools, Los Angeles, CA, USA)."
Hoebart C, Kiss A, Pilz PM, Szabo PL, Podesser BK, Fischer MJM, Heber S. TRPA1 as Target in Myocardial Infarction. Int J Mol Sci. 2023 Jan 28;24(3):2516. doi: 10.3390/ijms24032516. PMID: 36768836; PMCID: PMC9917254.
- Trimodality therapy versus perioperative chemotherapy in the management of locally advanced adenocarcinoma of the oesophagus and oesophagogastric junction (Neo-AEGIS): an open-label, randomised, phase 3 trial
-
"Randomisation sequences were generated using nQuery Advisor (version 6.01) using a permuted-block method to ensure a balance in sample size across the groups, with randomly permuted blocks of sizes two, four, six, and eight."
Reynolds JV, Preston SR, O'Neill B, Lowery MA, Baeksgaard L, Crosby T, Cunningham M, Cuffe S, Griffiths GO, Parker I, Risumlund SL, Roy R, Falk S, Hanna GB, Bartlett FR, Alvarez-Iglesias A, Achiam MP, Nilsson M, Piessen G, Ravi N, O'Toole D, Johnston C, McDermott RS, Turkington RC, Wahed S, Sothi S, Ford H, Wadley MS, Power D; Neo-AEGIS Investigators and Trial Group. Trimodality therapy versus perioperative chemotherapy in the management of locally advanced adenocarcinoma of the oesophagus and oesophagogastric junction (Neo-AEGIS): an open-label, randomised, phase 3 trial. Lancet Gastroenterol Hepatol. 2023 Nov;8(11):1015-1027. doi: 10.1016/S2468-1253(23)00243-1. Epub 2023 Sep 18. PMID: 37734399; PMCID: PMC10567579.
- The Abdominal Pain Unit (APU). Study protocol of a standardized and structured care pathway for patients with atraumatic abdominal pain in the emergency department: A stepped wedged cluster randomized controlled trial
-
"An a-priori power calculation based on the primary endpoints (acute pain score at discharge from ED, duration of treatment at the ED, patient satisfaction) with nQuery Advisor 7.0."
Altendorf MB, Möckel M, Schenk L, Fischer-Rosinsky A, Frick J, Helbig L, Horenkamp-Sonntag D, Huscher D, Lichtenberg L, Reinhold T, Schindel D, Stier B, Sydow H, Wu YN, Zimmermann G, Slagman A. The Abdominal Pain Unit (APU). Study protocol of a standardized and structured care pathway for patients with atraumatic abdominal pain in the emergency department: A stepped wedged cluster randomized controlled trial. PLoS One. 2022 Aug 24;17(8):e0273115. doi: 10.1371/journal.pone.0273115. PMID: 36001620; PMCID: PMC9401147.
- Efficacy and safety of biosimilar CT-P17 versus reference adalimumab in subjects with rheumatoid arthritis: 24-week results from a randomized study
-
"A sample size of 450 subjects (225 per treatment group) was determined to provide ≥ 80% statistical power to demonstrate equivalence of ACR20 response at week 24, using nQuery Adviser "
Kay J, Jaworski J, Wojciechowski R, Wiland P, Dudek A, Krogulec M, Jeka S, Zielinska A, Trefler J, Bartnicka-Maslowska K, Krajewska-Wlodarczyk M, Klimiuk PA, Lee SJ, Bae YJ, Yang GE, Yoo JK, Furst DE, Keystone E. Efficacy and safety of biosimilar CT-P17 versus reference adalimumab in subjects with rheumatoid arthritis: 24-week results from a randomized study. Arthritis Res Ther. 2021 Feb 5;23(1):51. doi: 10.1186/s13075-020-02394-7. PMID: 33546755; PMCID: PMC7863328.
- A Phase 3 Trial of l-Glutamine in Sickle Cell Disease
-
"The sample size was calculated with the use of the Wilcoxon rank-sum test for ordered categories and nQuery Advisor software, version 7.0 (Statistical Solutions). "Niihara Y, Miller ST, Kanter J, Lanzkron S, Smith WR, Hsu LL, Gordeuk VR, Viswanathan K, Sarnaik S, Osunkwo I, Guillaume E, Sadanandan S, et al., ; Investigators of the Phase 3 Trial of l-Glutamine in Sickle Cell Disease. A Phase 3 Trial of l-Glutamine in Sickle Cell Disease. N Engl J Med. 2018 Jul 19;379(3):226-235. doi: 10.1056/NEJMoa1715971. PMID: 30021096.
- Clinical Efficacy of Enzalutamide vs Bicalutamide Combined With Androgen Deprivation Therapy in Men With Metastatic Hormone-Sensitive Prostate Cancer
-
"Those assumptions would require 41 patients per group (total of 82 patients), as calculated from the nQuery Advisor statistical software version 7.0 (Statsols). "Vaishampayan UN, Heilbrun LK, Monk P, et al. Clinical Efficacy of Enzalutamide vs Bicalutamide Combined With Androgen Deprivation Therapy in Men With Metastatic Hormone-Sensitive Prostate Cancer: A Randomized Clinical Trial. JAMA Netw Open. 2021;4(1):e2034633. doi:10.1001/jamanetworkopen.2020.34633
- Differences in gait analysis and clinical outcome after TightRope® or screw fixation in acute syndesmosis rupture: study protocol for a prospective randomized pilot study
-
"Patients will be randomly assigned to one of two groups (intervention or control) using a computer-generated random block assignment in a 1:1 ratio using nQuery Advisor v7.0 software (Statsols, Cork, Ireland). "
Doll, J., Waizenegger, S., Bruckner, T. et al. Differences in gait analysis and clinical outcome after TightRope® or screw fixation in acute syndesmosis rupture: study protocol for a prospective randomized pilot study. Trials 21, 606 (2020). https://doi.org/10.1186/s13063-020-04550-5
- High efficacy of a dimeticone-based pediculicide following a brief application: in vitro assays and randomized controlled investigator-blinded clinical trial
-
"A sample size of 42 was required for a one group χ2-test comparing cure rate of 90% with a fixed limit of 70% (two-sided test; alpha-level of 0.05; power = 90%; software nQuery advisor 7.0; power = 80%: sample size = 34). "
Heukelbach, J., Wolf, D., Clark, J.M. et al. High efficacy of a dimeticone-based pediculicide following a brief application: in vitro assays and randomized controlled investigator-blinded clinical trial. BMC Dermatol 19, 14 (2019). https://doi.org/10.1186/s12895-019-0094-4 - Post-stroke treatment with argon attenuated brain injury, reduced brain inflammation and enhanced M2 microglia/macrophage polarization: a randomized controlled animal study
-
"The 6-point neuroscore from 24 h to d7 after reperfusion was determined as the primary outcome. Sample size was calculated based on prior studies [34, 35]. The calculation was performed using nQuery Advisor + nTerim 4.0 "
Liu, J., Nolte, K., Brook, G. et al. Post-stroke treatment with argon attenuated brain injury, reduced brain inflammation and enhanced M2 microglia/macrophage polarization: a randomized controlled animal study. Crit Care 23, 198 (2019). https://doi.org/10.1186/s13054-019-2493-7
- Phase II Evaluation of Aggressive Dose De-Escalation for Adjuvant Chemoradiotherapy in Human Papillomavirus–Associated Oropharynx Squamous Cell Carcinoma
-
"A sample size of 35 evaluable patients for each cohort was sufficient to estimate LRR of 20% or less with a two-sided 85% CI that would contain 10% (nQuery Advisor [version 6.01]; CIs for a single proportion) and had 85% power to detect a decrease in acute grade 3 or worse toxicity from 40% to 20% or less, assuming a one-sided significance level of .06."
Ma DJ, Price KA, Moore EJ, Patel SH, Hinni ML, Garcia JJ, Graner DE, Foster NR, Ginos B, Neben-Wittich M, Garces YI, Chintakuntlawar AV, Price DL, Olsen KD, Van Abel KM, Kasperbauer JL, Janus JR, Waddle M, Miller R, Shiraishi S, Foote RL. Phase II Evaluation of Aggressive Dose De-Escalation for Adjuvant Chemoradiotherapy in Human Papillomavirus-Associated Oropharynx Squamous Cell Carcinoma. J Clin Oncol. 2019 Aug 1;37(22):1909-1918. doi: 10.1200/JCO.19.00463. Epub 2019 Jun 4. Erratum in: J Clin Oncol. 2020 Apr 1;38(10):1118. PMID: 31163012; PMCID: PMC7098832.
- Prospective, Multicenter, Randomized Phase III Trial Evaluating the Impact of Lymphoscintigraphy as Part of Sentinel Node Biopsy in Early Breast Cancer: SenSzi (GBG80) Trial
-
"Noninferiority was defined as follows: one-sided 95% CI stratified according to focality and trial site for the difference (without LSG – with LSG) in the mean number of histologically detected SLNs should be greater than −0.27 (10% noninferiority margin). To obtain a power of 80%, a minimum of 1,102 evaluable patients (n = 551 per arm) was estimated using nQuery Advisor (v 6.02)."
Kuemmel S, Holtschmidt J, Gerber B, Von der Assen A, Heil J, Thill M, Krug D, Schem C, Denkert C, Lubitz J, Blohmer JU, Reinisch M, Hötzeldt M, Seither F, Nekljudova V, Schwidde I, Uhrhan K, Von Minckwitz G, Rezai M, Mulowski J, Loibl S, Kuehn T. Prospective, Multicenter, Randomized Phase III Trial Evaluating the Impact of Lymphoscintigraphy as Part of Sentinel Node Biopsy in Early Breast Cancer: SenSzi (GBG80) Trial. J Clin Oncol. 2019 Jun 10;37(17):1490-1498. doi: 10.1200/JCO.18.02092. Epub 2019 May 1. PMID: 31042410.
- A Randomized Controlled Trial: Regenerative Effects, Efficacy and Safety of Erythropoietin in Burn and Scalding Injuries
-
"A sample size of 49 in each group would, therefore, have 90% power to detect a difference in means of 4 days, assuming that the common SD is 6 days using an independent samples t-test with a 0.05 two-sided significance level (nQuery Advisor 7.0) "
Front. Pharmacol., 31 October 2018 | https://doi.org/10.3389/fphar.2018.00951
- Effect of Adjuvant Trastuzumab for a Duration of 9 Weeks vs 1 Year With Concomitant Chemotherapy for Early Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer The SOLD Randomized Clinical Trial
-
"The primary analysis was planned for when approximately 366 DFS events were reached or when the last patient entered was followed up for 2.0 years after randomization, whichever occurred first. The sample size was calculated using nQuery Advisor, version 6.0 (Statistical Solutions Ltd)."
Joensuu H, Fraser J, Wildiers H, et al. Effect of Adjuvant Trastuzumab for a Duration of 9 Weeks vs 1 Year With Concomitant Chemotherapy for Early Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: The SOLD Randomized Clinical Trial. JAMA Oncol. 2018;4(9):1199–1206. doi:10.1001/jamaoncol.2018.1380
- A Phase 3 Trial of l-Glutamine in Sickle Cell Disease
-
"The sample size was calculated with the use of the Wilcoxon rank-sum test for ordered categories and nQuery Advisor software, version 7.0 (Statistical Solutions). "Niihara Y, Miller ST, Kanter J, Lanzkron S, Smith WR, Hsu LL, Gordeuk VR, Viswanathan K, Sarnaik S, Osunkwo I, Guillaume E, Sadanandan S, et al., ; Investigators of the Phase 3 Trial of l-Glutamine in Sickle Cell Disease. A Phase 3 Trial of l-Glutamine in Sickle Cell Disease. N Engl J Med. 2018 Jul 19;379(3):226-235. doi: 10.1056/NEJMoa1715971. PMID: 30021096.
- Epidemiology and risk factors of extensively drug-resistant Pseudomonas aeruginosa infections
-
"Using NQuery Advisor version 5.0 to determine the prevalence of XDR-PA infections within 5% with a 95% level of confidence, a necessary evaluable sample size of 323 patients was calculated. As we estimated a non-evaluable rate of 20%, an enrollment of 388 patients was required."
Palavutitotai N, Jitmuang A, Tongsai S, Kiratisin P, Angkasekwinai N. Epidemiology and risk factors of extensively drug-resistant Pseudomonas aeruginosa infections. PLoS One. 2018 Feb 22;13(2):e0193431. doi: 10.1371/journal.pone.0193431. PMID: 29470531; PMCID: PMC5823452.
- PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock
-
"To allow for a withdrawal rate of 3%, 706 patients were recruited. The software used for sample-size calculation was nQuery Advisor, version 7.0 (Statistical Solutions)."
Holger Thiele, M.D., Ibrahim Akin, M.D., Marcus Sandri, M.D., Georg Fuernau, M.D., Suzanne de Waha, M.D., Roza Meyer-Saraei, Ph.D., Peter Nordbeck, M.D., Tobias Geisler, M.D., Ulf Landmesser, M.D., Carsten Skurk, M.D., Andreas Fach, M.D., Harald Lapp, M.D., et al., ; CULPRIT-SHOCK Investigators. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N Engl J Med. 2017 Dec 21;377(25):2419-2432. doi: 10.1056/NEJMoa1710261. Epub 2017 Oct 30. PMID: 29083953.
- Uninterrupted Dabigatran versus Warfarin for Ablation in Atrial Fibrillation
-
"The trial had a planned enrollment of at least 290 patients per treatment group undergoing ablation across 114 international sites. Calculations were performed with the use of nQuery Advisor, version 7.0 (Statistical Solutions). "
Seligman SJ. Uninterrupted Dabigatran versus Warfarin for Ablation in Atrial Fibrillation. N Engl J Med. 2017 Aug 3;377(5):495. doi: 10.1056/NEJMc1707247. PMID: 28767344.
- Peripheral Nerve Dysfunction in Middle-Aged Subjects Born with Thalidomide Embryopathy
-
"A sample size of 17 patients per group yields a power of at least 80% comparing two independent means at a two-sided significance level of 5% as long as the true difference is in excess of one standard deviation. The sample size / power calculation was carried out using nQuery Advisor version 7.0."
Nicotra A, Newman C, Johnson M, Eremin O, Friede T, Malik O, Nicholas R. Peripheral Nerve Dysfunction in Middle-Aged Subjects Born with Thalidomide Embryopathy. PLoS One. 2016 Apr 21;11(4):e0152902. doi: 10.1371/journal.pone.0152902. PMID: 27100829; PMCID: PMC4839770.
- Results of the TOP Study: Prospectively Randomized Multicenter Trial of an Ex Vivo Tacrolimus Rinse Before Transplantation in EDC Livers
-
"The power of the test was 80% at a significance level of 0.05. Therefore, sample size estimation (nQuery Advisor 6.1; Statistical Solutions, Saugus, MA) for 2 unpaired samples using the Wilcoxon rank-sum test with an expected dropout rate of 15% resulted in an estimated sample size of 86 patients (43 tacrolimus + HTK vs 43 HTK-only). "
Pratschke, Sebastian MD1; Arnold, Hannah MD1; Zollner, Alfred MD2; Heise, Michael MD3; Pascher, Andreas MD4; Schemmer, Peter MD5; Scherer, Marcus N. MD6; Bauer, Andreas MD7; Jauch, Karl-Walter MD1; Werner, Jens MD1; Guba, Markus MD1; Angele, Martin K. MD1 Results of the TOP Study: Prospectively Randomized Multicenter Trial of an Ex Vivo Tacrolimus Rinse Before Transplantation in EDC Livers, Transplantation Direct: June 2016 - Volume 2 - Issue 6 - p e76 doi: 10.1097/TXD.0000000000000588
- Effect of an Echinacea-Based Hot Drink Versus Oseltamivir in Influenza Treatment: A Randomized, Double-Blind, Double-Dummy, Multicenter, Noninferiority Clinical Trial
-
"Because a generalized Wilcoxon test was used to evaluate all primary parameters combined (Day 1, 5, and 10), a power of at least 80% was expected for the combined hypothesis as well. Sample size was calculated with nQuery Advisor 7.0 (lower confidence limit for difference in proportions)"
Karel Rauš, Stephan Pleschka, Peter Klein, Roland Schoop, Peter Fisher, Effect of an Echinacea-Based Hot Drink Versus Oseltamivir in Influenza Treatment: A Randomized, Double-Blind, Double-Dummy, Multicenter, Noninferiority Clinical Trial,Current Therapeutic Research,Volume 77,2015,Pages 66-72,ISSN 0011-393X,https://doi.org/10.1016/j.curtheres.2015.04.001.
- Efficacy of Kisspeptin-54 to Trigger Oocyte Maturation in Women at High Risk of Ovarian Hyperstimulation Syndrome (OHSS) During In Vitro Fertilization (IVF) Therapy
-
"Randomization was performed using printed lists that were prepared by the trial statistician using NQuery at the Imperial Clinical Trials Unit. Participants, embryologists, and the clinicians who carried out oocyte retrieval were all blinded to the dose allocation."
Ali Abbara, Channa N. Jayasena, Georgios Christopoulos, Shakunthala Narayanaswamy, Chioma Izzi-Engbeaya, Gurjinder M. K. Nijher, Alexander N. Comninos, Deborah Peters, Adam Buckley, Risheka Ratnasabapathy, Julia K. Prague, Rehan Salim, Stuart A. Lavery, Stephen R. Bloom, Matyas Szigeti, Deborah A. Ashby, Geoffrey H. Trew, Waljit S. Dhillo, Efficacy of Kisspeptin-54 to Trigger Oocyte Maturation in Women at High Risk of Ovarian Hyperstimulation Syndrome (OHSS) During In Vitro Fertilization (IVF) Therapy, The Journal of Clinical Endocrinology & Metabolism, Volume 100, Issue 9, 1 September 2015, Pages 3322–3331, https://doi.org/10.1210/jc.2015-2332
- A 1-year follow-up of an experimental study of a self-management arthritis programme with an added exercise component of clients with osteoarthritis of the knee
-
"Mean change (12 months minus baseline) and the effect size of the outcome measures were calculated by Mann-Whitney U test and nQuery Advisor 4.0."
Yip YB, Sit JW, Wong DY, Chong SY, Chung LH. A 1-year follow-up of an experimental study of a self-management arthritis programme with an added exercise component of clients with osteoarthritis of the knee. Psychol Health Med. 2008 Aug;13(4):402-14. doi: 10.1080/13548500701584030. PMID: 18825579.
- Statistical issues in the use of the comet assay
-
"Calculations derived from nQuery Advisor and based upon sample sizes for comparison between two groups with within-group SD of 1 unit. Two-sided test with α = 0.05."
David P. Lovell, Takashi Omori, Statistical issues in the use of the comet assay, Mutagenesis, Volume 23, Issue 3, May 2008, Pages 171–182, https://doi.org/10.1093/mutage/gen015
- A simple sample size formula for analysis of covariance in randomized clinical trials
-
"when the calculated power based on the design factor and the results of nQuery Advisor was 90% and 80%, respectively."
George F. Borma, *, Jaap Fransenb , Wim A.J.G. Lemmensa. Journal of Clinical Epidemiology 60 (2007) 1234e1238
- A refined in vitro model to study inflammatory responses in organotypic membrane culture of postnatal rat hippocampal slices
-
"In order to reduce the within-group variation we used lognormal distribution and carried out the statistical power analysis calculations using nQuery Advisor®, version 5.0 "
Huuskonen, J., Suuronen, T., Miettinen, R. et al. A refined in vitro model to study inflammatory responses in organotypic membrane culture of postnatal rat hippocampal slices. J Neuroinflammation 2, 25 (2005). https://doi.org/10.1186/1742-2094-2-25
nQueryが選ばれる理由
規制当局からの承認

FDA/EMA申請に求められる適切なサンプルサイズを、一貫性を保ちながら計算します
- 承認申請に認められるサンプルサイズ設定と算出方法
- サンプルサイズ計算方法の生成
- IQ/OQ手順を自動化
臨床試験のリスクとコストの低減
ベイズ統計アプローチとアダプティブ・デザインが試験デザインを最適化します
- ベイズ統計に基づいたサンプルサイズと検出力から「ポジティブ」な結果が得られる可能性を検討
- 統計的に有意な結果を保持しサンプルサイズを再算出
- 盲検下および非盲検下でのサンプルサイズ再計算
強力なサンプルサイズオプション
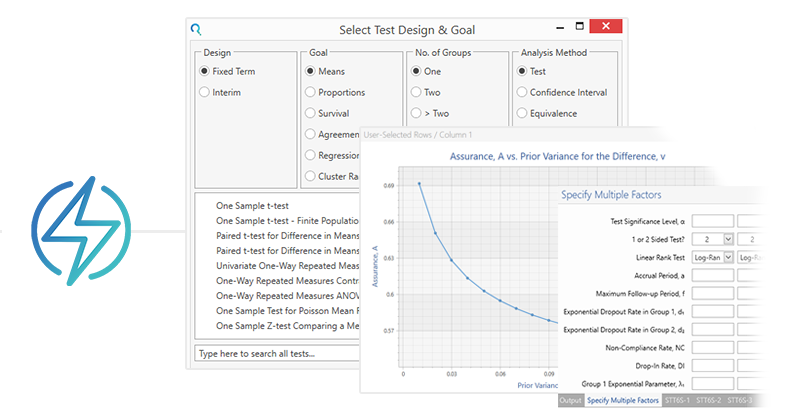
多くのシナリオに対するサンプルサイズを計算します
- 何百ものサンプルサイズと検出力を計算
- 科学的妥当性と予算的な側面から決定した最終的なサンプルサイズに合わせ、様々な"What-If"シナリオを実行(プロット機能)
- 複数の要因を特定する Specify Multiple Factors機能による計算の微調整
理解しやすく直感的な操作
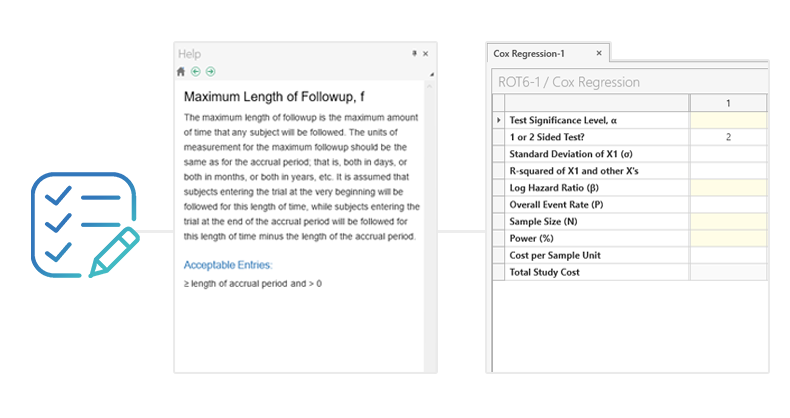
処理が速く分かりやすいスプレッドシート形式のインターフェイスで、コーディングの必要はありません
- 手動コーディング不要
- 統計ヘルプ機能
- カスタム可能なテーブル
ケーススタディ/事例紹介
nQueryで行う臨床試験の最適化

京都府立医科大学生物統計学講座
手良向聡教授
京都府立医科大学生物統計学講座の主任として、多くの医師主導臨床試験を先導している手良向聡教授もnQueryユーザーの1人です。臨床試験デザインの分野で35年以上の経験を有し、単群探索的臨床試験のベイズ流予測標本サイズデザインから、ステージII大腸癌に対する補助療法に焦点を当てた唯一のランダム化対照試験の計画・実施まで、手良向教授が携わったプロジェクトは多岐にわたります。
手良向教授が携わった臨床試験より、ステージIIの大腸癌手術後の試験計画策定や実施計画書作成の際にnQueryが使用された事例をケーススタディとして紹介しています。
世界中の研究者に評価されています
nQueryはサンプルサイズの設計および検出力分析ツールとしてだけではなく、臨床試験デザインのプラットフォームとして多くの研究者に利用されています。nQueryを使用した論文は、医学・ライフサイエンス分野のジャーナルに広く掲載されています。
nQueryは、臨床試験の完全な試験デザイン・プラットフォームです。実施する必要のある試験デザインがわかっていれば、サンプルサイズの計算は数分で完了します。SASやRで同じ計算をプログラムで行おうとすると、通常倍の時間がかかります。nQueryのドキュメントは非常に有用で、試験デザインを実施する上で必要な計算を理解し、明確なガイダンスを提供してくれます。
“nQuery is the complete trial design platform to make clinical
trials faster, less costly, and more successful. If the user knows the study design that needs to be implemented, then sample size calculation is a question of just minutes. Trying to programmatically do the same calculations in SAS or R usually takes more than 20 times longer. nQuery’s documentation is very helpful and provides very clear guidance to understand and implement the calculations that you need to perform."
Case Study1: Adaptive Designs in nQuery
Dr Luis Rojas
Executive Director Head Of Biostatistics At Target Health
20年ほどnQueryを使用しています。私にとってQueryは臨床試験デザインにおける標本サイズ設定の際に、最も信頼できるツールの1つです。複雑な計算を行うときに、統計ソフトウェアの信頼性はとても重要ですが、nQueryはその点をクリアしています。使いやすく、α消費関数などのグループ逐次デザインの棄却値を計算する際に役立っています。
"I have been using nQuery for about 20 years. Reliability is so important in statistical software when implementing complex calculations. nQuery is one of the most reliable tools for sample size calculation in clinical trial design. It is easy to use and very useful for calculating the critical values for group sequential designs such as alpha spending function."
Case Study2: Advancing patient care for Japan's most common cancer
Prof. Satoshi Teramukai
Chairman Of The Department Of Biostatistics At Kyoto Prefectural University
nQuery システム条件
nQuery 9.6 (2025/12/12 リリース)
nQueryのシステム条件は以下の通りです。インストール可能台数やライセンス許諾についてはFAQ:ライセンスについて をご参照下さい。
- OS: Microsoft Windows 11、 Microsoft .NET 8 を実行できる Windows 10 (v1607 以降)
- CPU: Intel/AMD (x64 ベース)
- RAM:2GB以上
- ハード空き容量:1GB以上
2015年以前のプロセッサまたはローエンドプロセッサは推奨しておりません。
nQuery製品カタログ
nQueryのデジタルカタログをダウンロードいただけます。